The U.S. Food and Drug Administration has approved Bristol-Myers Squibb’s product Daklinza (daclatasvir) for treatment of hepatitis C virus (HCV) genotype 3 infections. Daklinza is the first drug that has demonstrated safety and efficacy to treat genotype 3 HCV infections without requiring co-administration of interferon or ribavirin, two older FDA-approved drugs also used to treat HCV infection.
Hepatitis C is a viral disease that infects the liver and is transmitted through direct contact with infected blood and blood products. Up to 90 percent of persons infected with hepatitis C will not spontaneously clear the virus and will become chronically infected. Hepatitis C causes liver inflammation that can result in diminished liver function or liver failure. Most people infected with HCV will experience no symptoms of the disease until liver damage manifests, which may take several years after the initial infection. Some people with chronic HCV infection develop scarring leading to impaired liver function (cirrhosis) over many years, with complications such as bleeding, jaundice (yellowish eyes or skin), fluid accumulation in the abdomen, infections, or liver cancer. According to the World Health Organization, 20 percent of people with chronic hepatitis C will develop cirrhosis and, of those, about five to seven percent of patients may ultimately die of the consequences of infection.
Globally, some 150 million people infected with HCV, and among that cohort, an estimated 9 million are living with hepatitis C in the European Union (EU). According to the Centers for Disease Control and Prevention, an estimated 2.7 million Americans are infected with HCV, approximately 10 percent of which, are genotype 3.
“Today’s approval provides a new option for patients with genotype 3 HCV, including those patients who cannot tolerate ribavirin,” said Edward Cox, M.D., director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research at the July 24 Daklinza announcement.
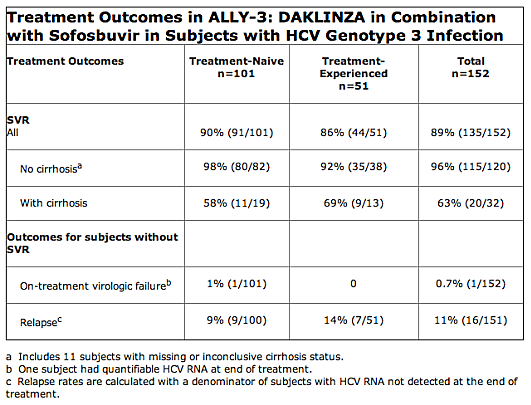
The FDA notes that Daklinza’s safety and efficacy of in combination with sofosbuvir were evaluated in an open-label clinical trial (ALLY-3) of 152 treatment-nave (n=101) and treatment-experienced (n-51) participants with chronic HCV genotype 3 infection. Most treatment-experienced subjects had failed prior treatment with peginterferon/ribavirin, but 7 subjects had been treated previously with a sofosbuvir regimen and 2 subjects with a regimen containing an investigational cyclophilin inhibitor. Participants received Daklinza 60 mg plus sofosbuvir 400 mg once daily for 12 weeks SVR and were monitored for 24 weeks post-treatment. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured. Outcomes in subjects without SVR in ALLY-3 are shown by patient population in the table below. Daklinza labeling carries a Limitations of Use statement to inform prescribers that sustained virologic response rates are reduced in HCV genotype 3 infected patients with cirrhosis.
Trial results showed 98 percent of the treatment-naive participants with no cirrhosis of the liver and 58 percent of the treatment-naive participants with cirrhosis achieved sustained virologic response. Among participants who were treatment-experienced, 92 percent with no cirrhosis of the liver and 69 percent with cirrhosis achieved sustained virologic response. Daklinza labeling carries a Limitations of Use statement to inform prescribers that sustained virologic response rates are reduced in HCV genotype 3 infected patients with cirrhosis.
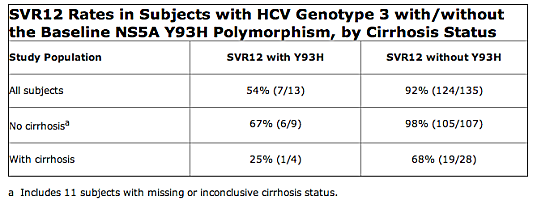
The FDA says based on resistance patterns observed in cell culture replicon studies and HCV genotype 3-infected subjects, cross-resistance between daclatasvir and other NS5A inhibitors is expected. Cross-resistance between daclatasvir and other classes of direct-acting antivirals is not expected. The impact of prior daclatasvir treatment experience on the efficacy of other NS5A inhibitors has not been studied. Conversely, the efficacy of Daklinza in combination with sofosbuvir has not been studied in subjects who have previously failed treatment with regimens that include an NS5A inhibitor.
Safety information was available for approximately 1,900 patients with HCV treated with the recommended dose of Daklinza in combination with other anti-HCV drugs in clinical trials. The most common side effects of Daklinza with sofosbuvir were fatigue (14%), headache (14%), nausea (8%) and diarrhea (5%). All adverse reactions were mild to moderate in severity. One subject experienced a serious adverse event that was considered unrelated to Daklinza, and no subjects discontinued therapy for adverse events. Transient, asymptomatic lipase elevations of greater than 3 times the upper limit of normal (ULN) were observed in 2% of subjects in ALLY-3. The optimal duration of Daklinza and sofosbuvir for patients with cirrhosis has not yet been established.
The recommended dosage of Daklinza is 60 mg, taken orally, once daily in combination with sofosbuvir for 12 weeks. Daklinza may be taken with or without food. Daklinza requires dosage modifications due to drug interactions. The dosage of Daklinza is reduced to 30 mg once daily when coadministered with strong CYP3A inhibitors using the 30 mg tablet. The dosage of Daklinza is increased to 90 mg once daily using an appropriate combination of tablets (three 30 mg tablets or one 60 mg and one 30 mg tablet) when coadministered with moderate CYP3A inducers.
Daklinza is contraindicated in combination with drugs that strongly induce CYP3A and, thus, may lead to lower exposure and loss of efficacy of Daklinza. Daklinza also carries a warning for patients and health care providers that serious slowing of the heart rate (symptomatic bradycardia) and cases requiring pacemaker intervention have been reported when amiodarone (an antiarrhythmic medication used to treat ventricular tachycardia or ventricular fibrillation) is co-administered with sofosbuvir in combination with another HCV direct-acting antiviral, including Daklinza. Co-administration of amiodarone with Daklinza in combination with sofosbuvir is not recommended.
Daklinza was reviewed under the FDA’s priority review program, which provides for an expedited review of drugs that treat serious conditions and, if approved, would provide significant improvement in safety or effectiveness.
The approve product label will be available soon at http://dailymed.nlm.nih.gov/dailymed/, or at drugs@fda
In last June 2014, Bristol-Myers Squibb also received positive Committee for Medicinal Products for Human Use (CHMP) opinion for Daklinza for treatment of Chronic Hepatitis C in the European Union, announcing that the CHMP of the European Medicines Agency (EMA) made a positive recommendation that Daklinza be granted approval for use in combination with other medicinal products for the treatment of chronic hepatitis C virus (HCV) infection in adults. Since then, Daklinza has already arrived in the Japanese and European markets in July 2014 and August 2014, respectively.
Bristol-Myers Squibb says ongoing and completed Daklinza studies have included more than 5,500 patients in a variety of all-oral regimens and with the current interferon-based standard of care. Across clinical studies, Daklinza-based regimens have been generally well tolerated with low rates of discontinuations across a range of patients.
The package insert data sheet for Daklinza can be viewed here:
http://packageinserts.bms.com/pi/pi_Daklinza.pdf
For more information, visit
http://www.bms.com
Sources:
The U.S. Food and Drug Administration
Bristol-Myers Squibb